Description and History of CFC's
CFC
- CFC stands for Chlorofluorocarbon which are a group of synthetic compounds that contain fluorine, chlorine, and carbon.
- The most common types of CFC's are trichlorofluoromethanes or CCl3F and dichlorodifluoromethane CCl2F2.
- The most common uses for these chemicals are used in refrigerants, making plastic foams for furniture, aerosol products, and insulation.
- They are able to change from liquid to gas or gas to liquid easily.
- CFC's are in a major category of man-made halo carbons which are formed when halogen gases such as fluorine and chlorine attach to carbon. They result in Ozone Depletion.
- CFC's were discovered by American Chemist Thomas Midgley in the 1930's. He later developed the CFC refrigerant, Freon while working for General Motors.
- CFC's drift in the environment until it reached the stratosphere where ultraviolet rays splits the chlorine molecule and attracts one of the three oxygen atoms of ozone and destroying it into oxygen.
- In 1978, the U.S. Government banned CFC aerosols as a result of reports and evidence of depletion of ozone from these man-made compounds.
- In 1988, DuPont Company, the largest producer of CFC's in the world, announced plans to phase out production of CFC's.
- Many protocols and agreements have been made (check The Human Impact) to stop use of CFC's around the world.
- Two exceptions are: 1)Use in propellant inhalers for asthma patients.
- 2) Use in methyl chloroform to clean O-ring seals for space shuttles.
- CFC's can last in the atmosphere for 50-200 years.
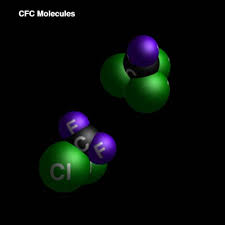 |
| The structure of a CFC |
 Cartoon of the ban of CFCs
Cartoon of the ban of CFCs