- Ozone is the Greek word for scent.
- Out of every 10 million air molecules, only three are ozone while two million are regular oxygen molecules.
- It is current labeled as O3 composed of two oxygen atoms and one regular molecule of oxygen.
- It has a molecular mass of 47.998.
- Pure Ozone is a blue gas.
- Ozone is a pollutant that can harm plant and animal tissues in the troposphere.
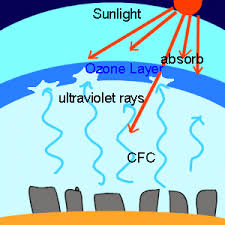
- In the stratosphere, it protects Ultraviolent Rays, but it depletes as a result.
- Highest regions of the Stratosphere contain 90% of all ozone.
- Dutch Chemist Von Marum was probably the first person to detect ozone gas sensorially.
- Christian Schonbein was the first chemist however to write about the discovery of ozone when he noticed a strange smell when conducting his experiments.
- He was also mentioned for being the first person to reearch the reactions mechanisms of ozone and organic matter.
- The first ozone generator was manufactured in Berlin by Von Siemens.
- Marcus Paul Otto received a doctorate for an essay on ozone. He later on started a specialized company for manufacture of ozone installations.
- The first-scale application of Ozone was in Oudshoorn, Netherlands.
- Ozone is created through photochemical reactions and by electrical discharges.
- Other reactions between gases such as nitrogen oxides and hydrocarbons create ozone and other ingridients of a pollutant called photochemcial smog.
Science Fair Project: Environmental Showcase by Paul Phan and Jordan Heffeler.
Description and History of Ozone
Ozone
Subscribe to:
Comments (Atom)